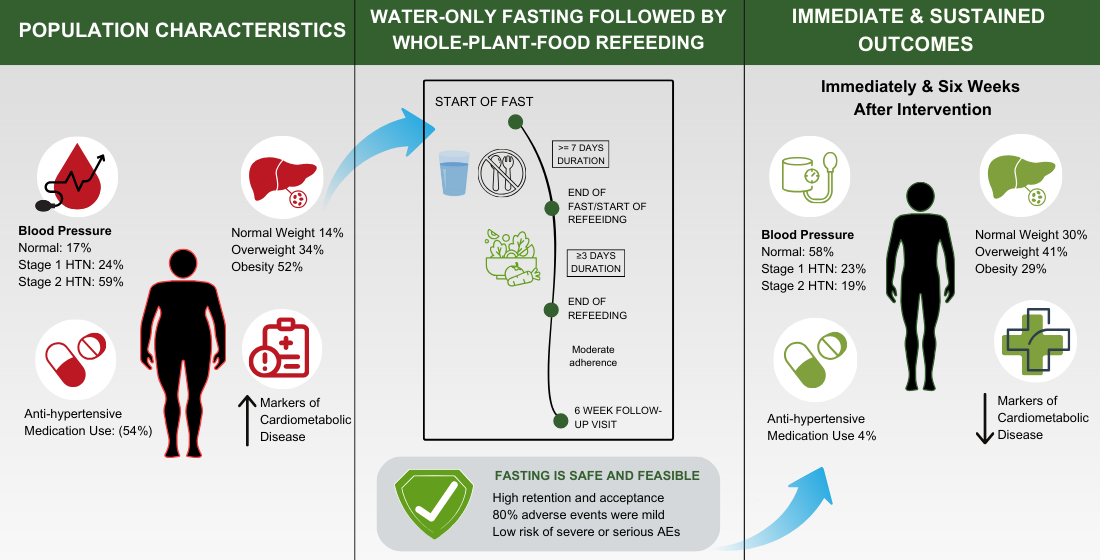
Abstract: Objective: This single-arm, pre–post interventional trial (clinicaltrials.gov, NCT04515095) investigates the safety, feasibility, and potential effectiveness of prolonged water-only fasting followed by a whole-plant-food diet in the long-term management of hypertension and other cardiometabolic disorders. Methods: Safety was assessed based on adverse events (AEs) that were recorded according to Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Feasibility was assessed based on retention rate, ability to complete minimal fast length, and intervention acceptability. Twenty-nine participants with stage 1 and 2 hypertension and without type 2 diabetes were enrolled from a residential fasting center. Results: Study retention was 100% at the end of the refeed and 93% at the six-week follow-up visit. Median (range) fasting and refeeding duration were 11 (7–40) and 5 (3–17) days, respectively, and 90% of participants were able to complete at least 7 days of fasting. The majority of AEs were mild (grade 1) and transient and there were no higher-grade or serious AEs. At the end of the intervention, median systolic/diastolic blood pressure had normalized to below 130/80 mmHg, body weight reduced by >5%, and anti-hypertensive medication was completely discontinued. These results were sustained for at least six weeks and potentially up to one year. Conclusions: Our data suggest that the intervention may be a feasible, well-tolerated, low-risk option for lowering and managing high blood pressure, excess body weight, and other cardiometabolic disorders in people with stage 1 and 2 hypertension.